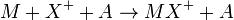| Home | Sources Directory | News Releases | Calendar | Articles | | Contact | |
Ion source
An ion source is an electro-magnetic device that is used to create charged particles. These are used primarily to form ions for mass spectrometers, optical emission spectrometers, particle accelerators, ion implanters and ion engines.
Contents |
[edit] Electron and chemical ionization
[edit] Electron ionization
Electron ionization is widely used in mass spectrometry, particularly for organic molecules. The gas phase reaction producing electron ionization is
where M is the atom of molecule being ionized, e ' is the electron, and 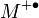 is the resulting ion.
is the resulting ion.
The electrons may be created by an arc discharge between a cathode and an anode.
[edit] Chemical ionization
Chemical ionization is a lower energy process than electron ionization.[1] The lower energy yields less fragmentation, and usually a simpler spectrum. A typical CI spectrum has an easily identifiable molecular ion.[2]
In a CI experiment, ions are produced through the collision of the analyte with ions of a reagent gas in the ion source. Some common reagent gases include: methane, ammonia, and isobutane. Inside the ion source, the reagent gas is present in large excess compared to the analyte. Electrons entering the source will preferentially ionize the reagent gas. The resultant collisions with other reagent gas molecules will create an ionization plasma. Positive and negative ions of the analyte are formed by reactions with this plasma. For example, protonation occurs by
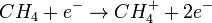 (primary ion formation),
(primary ion formation),
 (reagent ion formation),
(reagent ion formation),
![M + CH_5^+ \to CH_4 + [M + H]^+](http://upload.wikimedia.org/math/b/0/8/b080193dcb78a85fbec6fedb2cc0a0b7.png) (product ion formation, e.g. protonation).
(product ion formation, e.g. protonation).
[edit] Radioactive ion sources
A small piece of radioactive material, for instance 63Ni or 241Am, can be used to ionize a gas. This is used in ionization smoke detectors and ion mobility spectrometers.
[edit] Ion-attachment ionization
Ion-attachment ionization is similar to chemical ionization in which a cation is attached to the analyte molecule in a reactive collision:
Where M is the analyte molecule, X+ is the cation and A is a non-reacting collision partner.[3]
[edit] Gas discharge ion sources
These ion sources use a plasma source or electric discharge to create ions.
[edit] Inductively coupled plasma
Ions can be created in an inductively coupled plasma, which is a plasma source in which the energy is supplied by electrical currents which are produced by electromagnetic induction, that is, by time-varying magnetic fields.[4]
[edit] Microwave induced plasma
Microwaves are capable of exciting electrodeless gas discharges to create ions.
[edit] Glow discharge
Ions can be created in an electric glow discharge.
[edit] Spark ionization
Spark ionization is used to produce gas phase ions from a solid sample. When incorporated with a mass spectrometer the complete instrument is referred to as a spark ionization mass spectrometer or as a spark source mass spectrometer (SSMS).[5]
[edit] Closed drift ion sources
These ion sources use a radial magnetic field in an annular cavity in order to confine electrons for ionizing a gas. They are used for ion implantation and for space propulsion (Hall effect thrusters).
[edit] Desorption ionization
[edit] Aerosol laser desorption and ionization
In aerosol time-of-flight mass spectrometry, micrometer sized solid aerosol particles extracted from the atmosphere are simultaneously desorbed and ionized by a precisely timed laser pulse as they pass through the center of a time-of-flight ion extractor.[6][7]
[edit] Fast atom bombardment
In fast atom bombardment the analytes is mixed with a non-volatile chemical protection environment called a matrix and is bombarded under vacuum with a high energy (4000 to 10,000 electron volts) beam of atoms.[8] The atoms are typically from an inert gas such as argon or xenon. Common matrices include glycerol, thioglycerol, 3-nitrobenzyl alcohol (3-NBA), 18-Crown-6 ether, 2-nitrophenyloctyl ether, sulfolane, diethanolamine, and triethanolamine. This technique is similar to secondary ion mass spectrometry and plasma desorption mass spectrometry.
[edit] Field desorption
Field desorption refers to an ion source in which a high-potential electric field is applied to an emitter with a sharp surface, such as a razor blade, or more commonly, a filament from which tiny "whiskers" have formed.[9] This results in a very high electric field which can result in ionization of gaseous molecules of the analyte. Mass spectra produced by FI have little or no fragmentation. They are dominated by molecular radical cations M+. and less often, protonated molecules [M + H] + .
[edit] Liquid metal ion sources
In a Liquid metal ion source (LMIS), a metal (typically gallium) is heated to the liquid state and provided at the end of a capillary or a needle. Then a Taylor cone is formed under the application of a strong electric field. As the cone's tip get sharper, the electric field becomes stronger, until ions are produced by field evaporation. These ion sources are particularly used in ion implantation or in focused ion beam instruments.
[edit] Surface-enhanced laser desorption/ionization
Surface-enhanced laser desorption/ionization (SELDI) is a variant of MALDI that is used for the analysis of protein mixtures that uses a target modified to achieve biochemical affinity with the analyte compound.[10]
[edit] Spray ionization
[edit] Atmospheric pressure chemical ionization
Atmospheric pressure chemical ionization is a form of chemical ionization which takes place at atmospheric pressure.[11] A spray of solvent is heated to relatively high temperatures (above 400 degrees Celsius), sprayed with high flow rates of nitrogen and the entire aerosol cloud is subjected to a corona discharge that creates ions. APCI is not as "soft" an ionization technique as ESI.[12]
[edit] Atmospheric pressure photoionization
Atmospheric pressure photoionization uses a source of photons, usually a UV lamp, to ionize the elute in place of the corona pin used in APCI. A spray of solvent is heated to relatively high temperatures (above 400 degrees Celsius), sprayed with high flow rates of nitrogen for desolvation and the entire aerosol cloud is subjected to UV radiation to create ions.
[edit] Electrospray ionization
In electrospray ionization, a liquid is pushed through a very small, charged and usually metal, capillary.[13] This liquid contains the substance to be studied, the analyte, dissolved in a large amount of solvent, which is usually much more volatile than the analyte. Volatile acids, bases or buffers are often added to this solution too. The analyte exists as an ion in solution either in its anion or cation form. Because like charges repel, the liquid pushes itself out of the capillary and forms an aerosol, a mist of small droplets about 10 î�m across. The aerosol is at least partially produced by a process involving the formation of a Taylor cone and a jet from the tip of this cone. An uncharged carrier gas such as nitrogen is sometimes used to help nebulize the liquid and to help evaporate the neutral solvent in the droplets. As the solvent evaporates, the analyte molecules are forced closer together, repel each other and break up the droplets. This process is called Coulombic fission because it is driven by repulsive Coulombic forces between charged molecules. The process repeats until the analyte is free of solvent and is a bare ion. The ions observed are created by the addition of a proton (a hydrogen ion) and denoted [M + H] + , or of another cation such as sodium ion, [M + Na] + , or the removal of a proton, [M ' H] ' . Multiply-charged ions such as [M + 2H]2 + are often observed. For large macromolecules, there can be many charge states, occurring with different frequencies; the charge can be as great as [M + 25H]25 + , for example.
[edit] Probe electrospray ionization
Probe electrospray ionization (PESI) is a modified version of electrospray, where the capillary for sample solution transferring is replaced by a sharp-tipped solid needle with periodical motion.[14]
[edit] Sonic spray ionization
Sonic spray ionization is method for creating ions from a liquid solution, for example, a mixture of methanol and water.[15] A pneumatic nebulizer is used to turn the solution into a supersonic spray of small droplets. Ions are formed when the solvent evaporates and the statistically unbalanced charge distribution on the droplets leads to a net charge and complete desolvation results in the formation of ions.
[edit] Thermospray ionization
Thermospray is a form of atmospheric pressure ionization in mass spectrometry. It transfers ions from the liquid phase to the gas phase for analysis. It is particularly useful in liquid chromatography-mass spectrometry. [16]
[edit] Ambient ionization
In ambient ionization, ions are formed outside the mass spectrometer without sample preparation or separation.[17]
[edit] Desorption electrospray ionization
Desorption electrospray ionization uses an electrospray source to create charged droplets that are directed at a solid sample a few millimeters to a few centimeters away. The charged droplets pick up the sample through interaction with the surface and then form highly charged ions that can be sampled into a mass spectrometer.[18]
[edit] Direct analysis in real time
A Direct analysis in real time source operates by exposing the sample to a dry gas stream (typically helium or nitrogen) that contains long-lived electronically or vibronically excited neutral atoms or molecules (or "metastables"). Excited states are typically formed in the DART source by creating a glow discharge in a chamber through which the gas flows.
[edit] Matrix-assisted laser desorption electrospray ionization
Matrix-assisted laser desorption electrospray ionization (MALDESI)[19] is an atmospheric pressure ionization source for generation of multiply-charged ions. An ultraviolet or infrared laser is directed onto a solid or liquid sample containing the analyte of interest and matrix (UV = organic acid, IR = sacrificial analyte or water of hydration) desorbing neutral analyte molecules which are postionized by interaction with electrosprayed solvent droplets generating multiply charged ions.
[edit] Particle accelerators
In particle accelerators an ion source creates a particle beam at the beginning of the machine, the source. The technology to create ion sources for particle accelerators depends strongly on the type of particle that needs to be generated: electrons, protons, H- ion or a heavy ion.
Electrons are generated with an electron gun, and there are many varieties of these.
Protons are generated with a plasma-based device, like a duoplasmatron or a magnetron.
H- ions are generated with a magnetron or a Penning source. A magnetron consists of a central cylindrical cathode surrounded by an anode. The discharge voltage is typically greater than 150 V and the current drain is around 40 A. A magnetic field of about 0.2 tesla is parallel to the cathode axis. Hydrogen gas is introduced by a pulsed gas valve. Caesium is often used to lower the work function of the cathode, enhancing the amount of ions that are produced.
For a Penning source, a strong magnetic field parallel to the electric field of the sheath guides electrons and ions on cyclotron spirals from cathode to cathode. Fast H-minus ions are generated at the cathodes as in the magnetron. They are slowed down due to the charge exchange reaction as they migrate to the plasma aperture. This makes for a beam of ions that is colder than the ions obtained from a magnetron.
Heavy ions are generated with an electron cyclotron resonance ion source. The use of electron cyclotron resonance (ECR) ion sources for the production of intense beams of highly charged ions has immensely grown over the last decade. ECR ion sources are used as injectors into linear accelerators, Van-de-Graaff generators or cyclotrons in nuclear and elementary particle physics. In atomic and surface physics ECR ion sources deliver intense beams of highly charged ions for collision experiments or for the investigation of surfaces. For the highest charge states, however, Electron beam ion sources (EBIS) are needed. They can generate even bare ions of mid-heavy elements. The Electron beam ion trap (EBIT), based on the same principle, can produce up to bare uranium ions and can be used as an ion source as well.
[edit] Theory of Operation
Gas flows through the ion source between the anode and the cathode. A positive voltage is applied to the anode. This voltage, combined with the high magnetic field between the tips of the internal and external cathodes allow a plasma to start. Ions from the plasma are repelled by the anode electric field. This creates an ion beam.[20]
[edit] Ion source applications
- Surface cleaning and pretreatment for large area deposition
- Thin-film deposition
- Deposition of Thick Diamond-like carbon (DLC) Films
- Surface roughening of polymers for improved adhesion and/or biocompatibility[21]
[edit] References
- ^ Munson, M.S.B.; Field, F.H. J. Am. Chem. Soc. 1966, 88, 2621-2630. Chemical Ionization Mass Spectrometry. I. General Introduction.
- ^ de Hoffmann, Edmond; Vincent Stroobant (2003). Mass Spectrometry: Principles and Applications (Second ed.). Toronto: John Wiley & Sons, Ltd.. pp. 14. ISBN 0471485667.
- ^ "Lithium ion attachment mass spectrometry: Instrumentation and features". Review of Scientific Instruments.
- ^ A. Montaser and D. W. Golightly, eds. Inductively Coupled Plasmas in Analytical Atomic Spectrometry, VCH Publishers, Inc., New York, 1992.
- ^ H. E. Beske, A. Hurrle and K. P. Jochum (1981). "Part I. Principles of spark source mass spectrometry (SSMS)". Fresenius' Journal of Analytical Chemistry 309 (4): 258'261. doi:10.1007/BF00488596.
- ^ Carson, P; Neubauer, K; Johnston, M; Wexler, A (1995). "On-line chemical analysis of aerosols by rapid single-particle mass spectrometry". Journal of Aerosol Science 26: 535. doi:10.1016/0021-8502(94)00133-J.
- ^ Guazzotti, S; Coffee, K; Prather, K (2000). "Real time monitoring of size-resolved single particle chemistry during INDOEX-IFP 99". Journal of Aerosol Science 31: 182. doi:10.1016/S0021-8502(00)90189-7.
- ^ Morris HR, Panico M, Barber M, Bordoli RS, Sedgwick RD, Tyler A (1981). "Fast atom bombardment: a new mass spectrometric method for peptide sequence analysis". Biochem. Biophys. Res. Commun. 101 (2): 623'31. doi:10.1016/0006-291X(81)91304-8. PMID 7306100.
- ^ Beckey H.D. Field ionization mass spectrometry. Research/Development, 1969, 20(11), 26
- ^ Tang N, Tornatore P, Weinberger SR (2004). "Current developments in SELDI affinity technology". Mass spectrometry reviews 23 (1): 34'44. doi:10.1002/mas.10066. PMID 14625891.
- ^ Prakash C, Shaffer CL, Nedderman A (2007). "Analytical strategies for identifying drug metabolites". Mass spectrometry reviews 26 (3): 340'69. doi:10.1002/mas.20128. PMID 17405144.
- ^ Zaikin VG, Halket JM (2006). "Derivatization in mass spectrometry--8. Soft ionization mass spectrometry of small molecules". European journal of mass spectrometry (Chichester, England) 12 (2): 79'115. doi:10.1255/ejms.798. PMID 16723751.
- ^ Fenn, J. B.; Mann, M.; Meng, C. K.; Wong, S. F.; Whitehouse, C. M. (1990). "Electrospray Ionization-Principles and Practice". Mass Spectrometry Reviews 9 (1): 37'70. doi:10.1002/mas.1280090103.
- ^ Hiraoka K.; Nishidate K.; Mori K.; Asakawa D.; Suzuki S. (2007). "Development of probe electrospray using a solid needle". Rapid Communications in Mass Spectrometry 21 (18): 3139'3144. doi:10.1002/rcm.3201. PMID 17708527.
- ^ Hirabayashi A, Sakairi M, Koizumi H (1995). "Sonic spray mass spectrometry". Anal. Chem. 67 (17): 2878'82. doi:10.1021/ac00113a023. PMID 8779414.
- ^ Blakley, C. R.; Carmody, J. J.; Vestal, M. L. "Liquid Chromatograph-Mass Spectrometer for Analysis of Nonvolatile Samples" Analytical Chemistry 1980, 52, 1636-1641.
- ^ Cooks, R. Graham; Ouyang, Zheng; Takats, Zoltan; Wiseman, Justin M. (2006). "Ambient Mass Spectrometry". Science 311 (5767): 1566. doi:10.1126/science.1119426. PMID 16543450
- ^ Tak�¡ts Z, Wiseman JM, Cooks RG (2005). "Ambient mass spectrometry using desorption electrospray ionization (DESI): instrumentation, mechanisms and applications in forensics, chemistry, and biology". Journal of mass spectrometry : JMS 40 (10): 1261'75. doi:10.1002/jms.922. PMID 16237663.
- ^ Sampson JS, Hawkridge AM, Muddiman DC (2006). "Generation and detection of multiply-charged peptides and proteins by matrix-assisted laser desorption electrospray ionization (MALDESI) Fourier transform ion cyclotron resonance mass spectrometry". J. Am. Soc. Mass Spectrom. 17 (12): 1712'6. doi:10.1016/j.jasms.2006.08.003. PMID 16952462.
- ^ "Ion Beam Sources". Advanced Energy. Archived from the original on 2006-10-18. http://web.archive.org/web/20061018152802/http://www.advanced-energy.com/en/upload/File/Sources/SL-ION-230-02.pdf. Retrieved 2006-12-14.
- ^ "Ion Beam Source Technology". Advanced Energy. http://www.advanced-energy.com/en/Ion.html. Retrieved 2006-12-14.
[edit] See also
|
||||||||||||||||||||
|
SOURCES.COM is an online portal and directory for journalists, news media, researchers and anyone seeking experts, spokespersons, and reliable information resources. Use SOURCES.COM to find experts, media contacts, news releases, background information, scientists, officials, speakers, newsmakers, spokespeople, talk show guests, story ideas, research studies, databases, universities, associations and NGOs, businesses, government spokespeople. Indexing and search applications by Ulli Diemer and Chris DeFreitas.
For information about being included in SOURCES as a expert or spokesperson see the FAQ or use the online membership form. Check here for information about becoming an affiliate. For partnerships, content and applications, and domain name opportunities contact us.