| Home | Sources Directory | News Releases | Calendar | Articles | | Contact | |
Antibody
Antibodies (also known as immunoglobulins[1], abbreviated Ig) are gamma globulin proteins that are found in blood or other bodily fluids of vertebrates, and are used by the immune system to identify and neutralize foreign objects, such as bacteria and viruses. They are typically made of basic structural units'each with two large heavy chains and two small light chains'to form, for example, monomers with one unit, dimers with two units or pentamers with five units. Antibodies are produced by a kind of white blood cell called a plasma cell. There are several different types of antibody heavy chains, and several different kinds of antibodies, which are grouped into different isotypes based on which heavy chain they possess. Five different antibody isotypes are known in mammals, which perform different roles, and help direct the appropriate immune response for each different type of foreign object they encounter.[2]
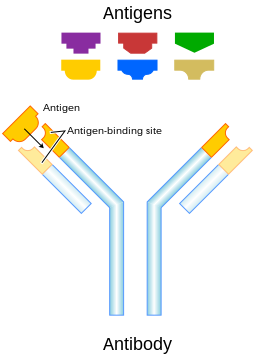
Though the general structure of all antibodies is very similar, a small region at the tip of the protein is extremely variable, allowing millions of antibodies with slightly different tip structures, or antigen binding sites, to exist. This region is known as the hypervariable region. Each of these variants can bind to a different target, known as an antigen.[3] This huge diversity of antibodies allows the immune system to recognize an equally wide variety of antigens. The unique part of the antigen recognized by an antibody is called the epitope. These epitopes bind with their antibody in a highly specific interaction, called induced fit, that allows antibodies to identify and bind only their unique antigen in the midst of the millions of different molecules that make up an organism. Recognition of an antigen by an antibody tags it for attack by other parts of the immune system. Antibodies can also neutralize targets directly by, for example, binding to a part of a pathogen that it needs to cause an infection.[4]
The large and diverse population of antibodies is generated by random combinations of a set of gene segments that encode different antigen binding sites (or paratopes), followed by random mutations in this area of the antibody gene, which create further diversity.[2][5] Antibody genes also re-organize in a process called class switching that changes the base of the heavy chain to another, creating a different isotype of the antibody that retains the antigen specific variable region. This allows a single antibody to be used by several different parts of the immune system. Production of antibodies is the main function of the humoral immune system.[6]
Contents |
[edit] Forms
Surface immunoglobulin (Ig) is attached to the membrane of the effector B cells by its transmembrane region, while antibodies are the secreted form of Ig and lack the trans membrane region so that antibodies can be secreted into the bloodstream and body cavities. As a result, surface Ig and antibodies are identical except for the transmembrane regions. Therefore, they are considered two forms of antibodies: soluble form or membrane-bound form (Parham 21-22).
The membrane-bound form of an antibody may be called a surface immunoglobulin (sIg) or a membrane immunoglobulin (mIg). It is part of the B cell receptor (BCR), which allows a B cell to detect when a specific antigen is present in the body and triggers B cell activation.[7] The BCR is composed of surface-bound IgD or IgM antibodies and associated Ig-î� and Ig-î� heterodimers, which are capable of signal transduction.[8] A typical human B cell will have 50,000 to 100,000 antibodies bound to its surface.[8] Upon antigen binding, they cluster in large patches, which can exceed 1 micrometer in diameter, on lipid rafts that isolate the BCRs from most other cell signaling receptors.[8] These patches may improve the efficiency of the cellular immune response.[9] In humans, the cell surface is bare around the B cell receptors for several thousand ångstroms,[8] which further isolates the BCRs from competing influences.
[edit] Isotypes
| Name | Types | Description | Antibody Complexes |
| IgA | 2 | Found in mucosal areas, such as the gut, respiratory tract and urogenital tract, and prevents colonization by pathogens.[10] Also found in saliva, tears, and breast milk. |  |
| IgD | 1 | Functions mainly as an antigen receptor on B cells that have not been exposed to antigens.[11] It has been shown to activate basophils and mast cells to produce antimicrobial factors.[12] | |
| IgE | 1 | Binds to allergens and triggers histamine release from mast cells and basophils, and is involved in allergy. Also protects against parasitic worms.[6] | |
| IgG | 4 | In its four forms, provides the majority of antibody-based immunity against invading pathogens.[6] The only antibody capable of crossing the placenta to give passive immunity to fetus. | |
| IgM | 1 | Expressed on the surface of B cells and in a secreted form with very high avidity. Eliminates pathogens in the early stages of B cell mediated (humoral) immunity before there is sufficient IgG.[6][11] |
Antibodies can come in different varieties known as isotypes or classes. In placental mammals there are five antibody isotypes known as IgA, IgD, IgE, IgG and IgM. They are each named with an "Ig" prefix that stands for immunoglobulin, another name for antibody, and differ in their biological properties, functional locations and ability to deal with different antigens, as depicted in the table.[13]
The antibody isotype of a B cell changes during cell development and activation. Immature B cells, which have never been exposed to an antigen, are known as naïve B cells and express only the IgM isotype in a cell surface bound form. B cells begin to express both IgM and IgD when they reach maturity'the co-expression of both these immunoglobulin isotypes renders the B cell 'mature' and ready to respond to antigen.[14] B cell activation follows engagement of the cell bound antibody molecule with an antigen, causing the cell to divide and differentiate into an antibody producing cell called a plasma cell. In this activated form, the B cell starts to produce antibody in a secreted form rather than a membrane-bound form. Some daughter cells of the activated B cells undergo isotype switching, a mechanism that causes the production of antibodies to change from IgM or IgD to the other antibody isotypes, IgE, IgA or IgG, that have defined roles in the immune system.
[edit] Structure
Antibodies are heavy (~150 kDa) globular plasma proteins. They have sugar chains added to some of their amino acid residues.[15] In other words, antibodies are glycoproteins. The basic functional unit of each antibody is an immunoglobulin (Ig) monomer (containing only one Ig unit); secreted antibodies can also be dimeric with two Ig units as with IgA, tetrameric with four Ig units like teleost fish IgM, or pentameric with five Ig units, like mammalian IgM.[16]
The variable parts of an antibody are its V regions, and the constant part is its C region.
[edit] Immunoglobulin domains
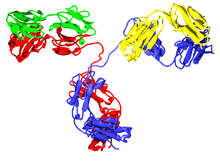
The Ig monomer is a "Y"-shaped molecule that consists of four polypeptide chains; two identical heavy chains and two identical light chains connected by disulfide bonds.[13] Each chain is composed of structural domains called immunoglobulin domains. These domains contain about 70-110 amino acids and are classified into different categories (for example, variable or IgV, and constant or IgC) according to their size and function.[17] They have a characteristic immunoglobulin fold in which two beta sheets create a 'sandwich' shape, held together by interactions between conserved cysteines and other charged amino acids.
[edit] Heavy chain
There are five types of mammalian Ig heavy chain denoted by the Greek letters: î�, î�, î�, î�, and î�.[3] The type of heavy chain present defines the class of antibody; these chains are found in IgA, IgD, IgE, IgG, and IgM antibodies, respectively.[4] Distinct heavy chains differ in size and composition; î� and î� contain approximately 450 amino acids, while î� and î� have approximately 550 amino acids.[3]
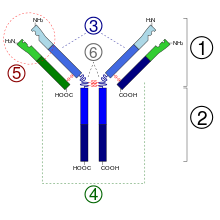
2. Fc region
3. Heavy chain with one variable (VH) domain followed by a constant domain (CH1), a hinge region, and two more constant (CH2 and CH3) domains.
4. Light chain with one variable (VL) and one constant (CL) domain
5. Antigen binding site (paratope)
6. Hinge regions.
Each heavy chain has two regions, the constant region and the variable region. The constant region is identical in all antibodies of the same isotype, but differs in antibodies of different isotypes. Heavy chains î�, î� and î� have a constant region composed of three tandem (in a line) Ig domains, and a hinge region for added flexibility;[13] heavy chains î� and î� have a constant region composed of four immunoglobulin domains.[3] The variable region of the heavy chain differs in antibodies produced by different B cells, but is the same for all antibodies produced by a single B cell or B cell clone. The variable region of each heavy chain is approximately 110 amino acids long and is composed of a single Ig domain.
[edit] Light chain
In mammals there are two types of immunoglobulin light chain, which are called lambda (î») and kappa (î�).[3] A light chain has two successive domains: one constant domain and one variable domain. The approximate length of a light chain is 211 to 217 amino acids.[3] Each antibody contains two light chains that are always identical; only one type of light chain, î� or î», is present per antibody in mammals. Other types of light chains, such as the iota (î�) chain, are found in lower vertebrates like Chondrichthyes and Teleostei.
[edit] CDRs, Fv, Fab and Fc Regions
Some parts of an antibody have unique functions. The arms of the Y, for example, contain the sites that can bind two antigens (in general identical) and, therefore, recognize specific foreign objects. This region of the antibody is called the Fab (fragment, antigen binding) region. It is composed of one constant and one variable domain from each heavy and light chain of the antibody.[18] The paratope is shaped at the amino terminal end of the antibody monomer by the variable domains from the heavy and light chains. The variable domain is also referred to as the FV region and is the most important region for binding to antigens. More specifically variable loops, three each on the light (VL) and heavy (VH) chains are responsible for binding to the antigen. These loops are referred to as the complementarity determining regions (CDRs). In the framework of the immune network theory, CDRs are also called idiotypes. According to immune network theory, the adaptive immune system is regulated by interactions between idiotypes.
The base of the Y plays a role in modulating immune cell activity. This region is called the Fc (Fragment, crystallizable) region, and is composed of two heavy chains that contribute two or three constant domains depending on the class of the antibody.[3] By binding to specific proteins the Fc region ensures that each antibody generates an appropriate immune response for a given antigen.[19] The Fc region also binds to various cell receptors, such as Fc receptors, and other immune molecules, such as complement proteins. By doing this, it mediates different physiological effects including opsonization, cell lysis, and degranulation of mast cells, basophils and eosinophils.[13][20]
[edit] Function
Activated B cells differentiate into either antibody-producing cells called plasma cells that secrete soluble antibody or memory cells that survive in the body for years afterward in order to allow the immune system to remember an antigen and respond faster upon future exposures.[21]
At the prenatal and neonatal stages of life, the presence of antibodies is provided by passive immunization from the mother. Early endogenous antibody production varies for different kinds of antibodies, and usually appear within the first years of life. Since antibodies exist freely in the bloodstream, they are said to be part of the humoral immune system. Circulating antibodies are produced by clonal B cells that specifically respond to only one antigen (an example is a virus capsid protein fragment). Antibodies contribute to immunity in three ways: they prevent pathogens from entering or damaging cells by binding to them; they stimulate removal of pathogens by macrophages and other cells by coating the pathogen; and they trigger destruction of pathogens by stimulating other immune responses such as the complement pathway.[22]

[edit] Activation of complement
Antibodies that bind to surface antigens on, for example, a bacterium attract the first component of the complement cascade with their Fc region and initiate activation of the "classical" complement system.[22] This results in the killing of bacteria in two ways.[6] First, the binding of the antibody and complement molecules marks the microbe for ingestion by phagocytes in a process called opsonization; these phagocytes are attracted by certain complement molecules generated in the complement cascade. Secondly, some complement system components form a membrane attack complex to assist antibodies to kill the bacterium directly.[23]
[edit] Activation of effector cells
To combat pathogens that replicate outside cells, antibodies bind to pathogens to link them together, causing them to agglutinate. Since an antibody has at least two paratopes it can bind more than one antigen by binding identical epitopes carried on the surfaces of these antigens. By coating the pathogen, antibodies stimulate effector functions against the pathogen in cells that recognize their Fc region.[6]
Those cells which recognize coated pathogens have Fc receptors which, as the name suggests, interacts with the Fc region of IgA, IgG, and IgE antibodies. The engagement of a particular antibody with the Fc receptor on a particular cell triggers an effector function of that cell; phagocytes will phagocytose, mast cells and neutrophils will degranulate, natural killer cells will release cytokines and cytotoxic molecules; that will ultimately result in destruction of the invading microbe. The Fc receptors are isotype-specific, which gives greater flexibility to the immune system, invoking only the appropriate immune mechanisms for distinct pathogens.[3]
[edit] Natural antibodies
Humans and higher primates also produce 'natural antibodies' which are present in serum before viral infection. Natural antibodies have been defined as antibodies that are produced without any previous infection, vaccination, other foreign antigen exposure or passive immunization. These antibodies can activate the classical complement pathway leading to lysis of enveloped virus particles long before the adaptive immune response is activated. Many natural antibodies are directed against the disaccharide galactose î�(1,3)-galactose (î�-Gal), which is found as a terminal sugar on glycosylated cell surface proteins, and generated in response to production of this sugar by bacteria contained in the human gut.[24] Rejection of xenotransplantated organs is thought to be, in part, the result of natural antibodies circulating in the serum of the recipient binding to î�-Gal antigens expressed on the donor tissue.[25]
[edit] Immunoglobulin diversity
Virtually all microbes can trigger an antibody response. Successful recognition and eradication of many different types of microbes requires diversity among antibodies; their amino acid composition varies allowing them to interact with many different antigens.[26] It has been estimated that humans generate about 10 billion different antibodies, each capable of binding a distinct epitope of an antigen.[27] Although a huge repertoire of different antibodies is generated in a single individual, the number of genes available to make these proteins is limited by the size of the human genome. Several complex genetic mechanisms have evolved that allow vertebrate B cells to generate a diverse pool of antibodies from a relatively small number of antibody genes.[28]
[edit] Domain variability
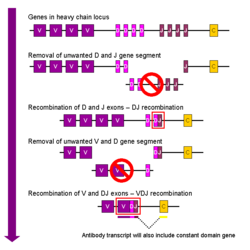
The region (locus) of a chromosome that encodes an antibody is large and contains several distinct genes for each domain of the antibody'the locus containing heavy chain genes (IGH@) is found on chromosome 14, and the loci containing lambda and kappa light chain genes (IGL@ and IGK@) are found on chromosomes 22 and 2 in humans. One of these domains is called the variable domain, which is present in each heavy and light chain of every antibody, but can differ in different antibodies generated from distinct B cells. Differences, between the variable domains, are located on three loops known as hypervariable regions (HV-1, HV-2 and HV-3) or complementarity determining regions (CDR1, CDR2 and CDR3). CDRs are supported within the variable domains by conserved framework regions. The heavy chain locus contains about 65 different variable domain genes that all differ in their CDRs. Combining these genes with an array of genes for other domains of the antibody generates a large cavalry of antibodies with a high degree of variability. This combination is called V(D)J recombination discussed below.[29]
[edit] V(D)J recombination
Somatic recombination of immunoglobulins, also known as V(D)J recombination, involves the generation of a unique immunoglobulin variable region. The variable region of each immunoglobulin heavy or light chain is encoded in several pieces'known as gene segments. These segments are called variable (V), diversity (D) and joining (J) segments.[28] V, D and J segments are found in Ig heavy chains, but only V and J segments are found in Ig light chains. Multiple copies of the V, D and J gene segments exist, and are tandemly arranged in the genomes of mammals. In the bone marrow, each developing B cell will assemble an immunoglobulin variable region by randomly selecting and combining one V, one D and one J gene segment (or one V and one J segment in the light chain). As there are multiple copies of each type of gene segment, and different combinations of gene segments can be used to generate each immunoglobulin variable region, this process generates a huge number of antibodies, each with different paratopes, and thus different antigen specificities.[2]
After a B cell produces a functional immunoglobulin gene during V(D)J recombination, it cannot express any other variable region (a process known as allelic exclusion) thus each B cell can produce antibodies containing only one kind of variable chain.[3][30]
[edit] Somatic hypermutation and affinity maturation
- For more details on this topic, see Somatic hypermutation and Affinity maturation
Following activation with antigen, B cells begin to proliferate rapidly. In these rapidly dividing cells, the genes encoding the variable domains of the heavy and light chains undergo a high rate of point mutation, by a process called somatic hypermutation (SHM). SHM results in approximately one nucleotide change per variable gene, per cell division.[5] As a consequence, any daughter B cells will acquire slight amino acid differences in the variable domains of their antibody chains.
This serves to increase the diversity of the antibody pool and impacts the antibody's antigen-binding affinity.[31] Some point mutations will result in the production of antibodies that have a weaker interaction (low affinity) with their antigen than the original antibody, and some mutations will generate antibodies with a stronger interaction (high affinity).[32] B cells that express high affinity antibodies on their surface will receive a strong survival signal during interactions with other cells, whereas those with low affinity antibodies will not, and will die by apoptosis.[32] Thus, B cells expressing antibodies with a higher affinity for the antigen will outcompete those with weaker affinities for function and survival. The process of generating antibodies with increased binding affinities is called affinity maturation. Affinity maturation occurs in mature B cells after V(D)J recombination, and is dependent on help from helper T cells.[33]
[edit] Class switching
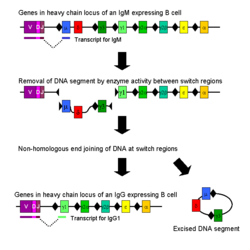
Isotype or class switching is a biological process occurring after activation of the B cell, which allows the cell to produce different classes of antibody (IgA, IgE, or IgG).[2] The different classes of antibody, and thus effector functions, are defined by the constant (C) regions of the immunoglobulin heavy chain. Initially, naïve B cells express only cell-surface IgM and IgD with identical antigen binding regions. Each isotype is adapted for a distinct function, therefore, after activation, an antibody with a IgG, IgA, or IgE effector function might be required to effectively eliminate an antigen. Class switching allows different daughter cells from the same activated B cell to produce antibodies of different isotypes. Only the constant region of the antibody heavy chain changes during class switching; the variable regions, and therefore antigen specificity, remain unchanged. Thus the progeny of a single B cell can produce antibodies, all specific for the same antigen, but with the ability to produce the effector function appropriate for each antigenic challenge. Class switching is triggered by cytokines; the isotype generated depends on which cytokines are present in the B cell environment.[34]
Class switching occurs in the heavy chain gene locus by a mechanism called class switch recombination (CSR). This mechanism relies on conserved nucleotide motifs, called switch (S) regions, found in DNA upstream of each constant region gene (except in the î�-chain). The DNA strand is broken by the activity of a series of enzymes at two selected S-regions.[35][36] The variable domain exon is rejoined through a process called non-homologous end joining (NHEJ) to the desired constant region (î�, î� or î�). This process results in an immunoglobulin gene that encodes an antibody of a different isotype.[37]
[edit] Medical applications
[edit] Disease diagnosis and therapy
Detection of particular antibodies is a very common form of medical diagnostics, and applications such as serology depend on these methods.[38] For example, in biochemical assays for disease diagnosis,[39] a titer of antibodies directed against Epstein-Barr virus or Lyme disease is estimated from the blood. If those antibodies are not present, either the person is not infected, or the infection occurred a very long time ago, and the B cells generating these specific antibodies have naturally decayed. In clinical immunology, levels of individual classes of immunoglobulins are measured by nephelometry (or turbidimetry) to characterize the antibody profile of patient.[40] Elevations in different classes of immunoglobulins are sometimes useful in determining the cause of liver damage in patients whom the diagnosis is unclear.[4] For example, elevated IgA indicates alcoholic cirrhosis, elevated IgM indicates viral hepatitis and primary biliary cirrhosis, while IgG is elevated in viral hepatitis, autoimmune hepatitis and cirrhosis. Autoimmune disorders can often be traced to antibodies that bind the body's own epitopes; many can be detected through blood tests. Antibodies directed against red blood cell surface antigens in immune mediated hemolytic anemia are detected with the Coombs test.[41] The Coombs test is also used for antibody screening in blood transfusion preparation and also for antibody screening in antenatal women.[41] Practically, several immunodiagnostic methods based on detection of complex antigen-antibody are used to diagnose infectious diseases, for example ELISA, immunofluorescence, Western blot, immunodiffusion, immunoelectrophoresis, and Magnetic immunoassay. Antibodies raised against Human chorionic gonadotropin are used in over the counter pregnancy tests. Targeted monoclonal antibody therapy is employed to treat diseases such as rheumatoid arthritis,[42] multiple sclerosis,[43] psoriasis,[44] and many forms of cancer including non-Hodgkin's lymphoma,[45] colorectal cancer, head and neck cancer and breast cancer.[46] Some immune deficiencies, such as X-linked agammaglobulinemia and hypogammaglobulinemia, result in partial or complete lack of antibodies.[47] These diseases are often treated by inducing a short term form of immunity called passive immunity. Passive immunity is achieved through the transfer of ready-made antibodies in the form of human or animal serum, pooled immunoglobulin or monoclonal antibodies, into the affected individual.[48]
[edit] Prenatal therapy
Rhesus factor, also known as Rhesus D (RhD) antigen, is an antigen found on red blood cells; individuals that are Rhesus-positive (Rh+) have this antigen on their red blood cells and individuals that are Rhesus-negative (Rh') do not. During normal childbirth, delivery trauma or complications during pregnancy, blood from a fetus can enter the mother's system. In the case of an Rh-incompatible mother and child, consequential blood mixing may sensitize an Rh- mother to the Rh antigen on the blood cells of the Rh+ child, putting the remainder of the pregnancy, and any subsequent pregnancies, at risk for hemolytic disease of the newborn.[49]
Rho(D) immune globulin antibodies are specific for human Rhesus D (RhD) antigen.[50] Anti-RhD antibodies are administered as part of a prenatal treatment regimen to prevent sensitization that may occur when a Rhesus-negative mother has a Rhesus-positive fetus. Treatment of a mother with Anti-RhD antibodies prior to and immediately after trauma and delivery destroys Rh antigen in the mother's system from the fetus. Importantly, this occurs before the antigen can stimulate maternal B cells to "remember" Rh antigen by generating memory B cells. Therefore, her humoral immune system will not make anti-Rh antibodies, and will not attack the Rhesus antigens of the current or subsequent babies. Rho(D) Immune Globulin treatment prevents sensitization that can lead to Rh disease, but does not prevent or treat the underlying disease itself.[50]
[edit] Research applications

Specific antibodies are produced by injecting an antigen into a mammal, such as a mouse, rat or rabbit for small quantities of antibody, or goat, sheep, or horse for large quantities of antibody. Blood isolated from these animals contains polyclonal antibodies'multiple antibodies that bind to the same antigen'in the serum, which can now be called antiserum. Antigens are also injected into chickens for generation of polyclonal antibodies in egg yolk.[51] To obtain antibody that is specific for a single epitope of an antigen, antibody-secreting lymphocytes are isolated from the animal and immortalized by fusing them with a cancer cell line. The fused cells are called hybridomas, and will continually grow and secrete antibody in culture. Single hybridoma cells are isolated by dilution cloning to generate cell clones that all produce the same antibody; these antibodies are called monoclonal antibodies.[52] Polyclonal and monoclonal antibodies are often purified using Protein A/G or antigen-affinity chromatography.[53]
In research, purified antibodies are used in many applications. They are most commonly used to identify and locate intracellular and extracellular proteins. Antibodies are used in flow cytometry to differentiate cell types by the proteins they express; different types of cell express different combinations of cluster of differentiation molecules on their surface, and produce different intracellular and secretable proteins.[54] They are also used in immunoprecipitation to separate proteins and anything bound to them (co-immunoprecipitation) from other molecules in a cell lysate,[55] in Western blot analyses to identify proteins separated by electrophoresis,[56] and in immunohistochemistry or immunofluorescence to examine protein expression in tissue sections or to locate proteins within cells with the assistance of a microscope.[54][57] Proteins can also be detected and quantified with antibodies, using ELISA and ELISPOT techniques.[58][59]
[edit] Structure prediction
The importance of antibodies in health care and the biotechnology industry demands knowledge of their structures at high resolution. This information is used for protein engineering, modifying the antigen binding affinity, and identifying an epitope, of a given antibody. X-ray crystallography is one commonly used method for determining antibody structures. However, crystallizing an antibody is often laborious and time consuming. Computational approaches can provide a cheaper faster alternative to crystallography, but their results are more equivocal since they do not produce empirical structures. Online web servers such as Web Antibody Modeling (WAM)[60] and Prediction of Immunoglobulin Structure (PIGS)[61] enables computational modeling of antibody variable regions. Rosetta Antibody is a novel antibody FV region structure prediction server, which incorporates sophisticated techniques to minimize CDR loops and optimize the relative orientation of the light and heavy chains, as well as homology models that predict successful docking of antibodies with their unique antigen.[62]
[edit] History
The first use of the term "antibody" occurred in a text by Paul Ehrlich. The term Antikörper (the German word for antibody) appears in the conclusion of his article "Experimental Studies on Immunity", published in October 1891, which states that "if two substances give rise to two different antikörper, then they themselves must be different".[63]. However, the term was not accepted immediately and several other terms for antibody were proposed; these included Immunkörper, Amboceptor, Zwischenkörper, substance sensibilisatrice, copula, Desmon, philocytase, fixateur, and Immunisin.[63] The word antibody has formal analogy to the word antitoxin and a similar concept to Immunkörper.[63]

The study of antibodies began in 1890 when Emil von Behring and Kitasato Shibasabur� described antibody activity against diphtheria and tetanus toxins. Behring and Kitasato put forward the theory of humoral immunity, proposing that a mediator in serum could react with a foreign antigen.[67][68] Their idea prompted Paul Ehrlich to propose the side chain theory for antibody and antigen interaction in 1897, when he hypothesized that receptors (described as 'side chains') on the surface of cells could bind specifically to toxins ' in a "lock-and-key" interaction ' and that this binding reaction was the trigger for the production of antibodies.[69] Other researchers believed that antibodies existed freely in the blood and, in 1904, Almroth Wright suggested that soluble antibodies coated bacteria to label them for phagocytosis and killing; a process that he named opsoninization.[70]
In the 1920s, Michael Heidelberger and Oswald Avery observed that antigens could be precipitated by antibodies and went on to show that antibodies were made of protein.[71] The biochemical properties of antigen-antibody binding interactions were examined in more detail in the late 1930s by John Marrack.[72] The next major advance was in the 1940s, when Linus Pauling confirmed the lock-and-key theory proposed by Ehrlich by showing that the interactions between antibodies and antigens depended more on their shape than their chemical composition.[73] In 1948, Astrid Fagreaus discovered that B cells, in the form of plasma cells, were responsible for generating antibodies.[74]
Further work concentrated on characterizing the structures of the antibody proteins. A major advance in these structural studies was the discovery in the early 1960s by Gerald Edelman and Joseph Gally of the antibody light chain,[75] and their realization that this protein was the same as the Bence-Jones protein described in 1845 by Henry Bence Jones.[76] Edelman went on to discover that antibodies are composed of disulfide bond-linked heavy and light chains. Around the same time, antibody-binding (Fab) and antibody tail (Fc) regions of IgG were characterized by Rodney Porter.[77] Together, these scientists deduced the structure and complete amino acid sequence of IgG, a feat for which they were jointly awarded the 1972 Nobel Prize in Physiology or Medicine.[77] While most of these early studies focused on IgM and IgG, other immunoglobulin isotypes were identified in the 1960s: Thomas Tomasi discovered secretory antibody (IgA)[78] and David Rowe[disambiguation needed] and John Fahey[disambiguation needed] identified IgD,[79] and IgE was identified by Kikishige Ishizaka and Teruki Ishizaka as a class of antibodies involved in allergic reactions.[80] Genetic studies identifying the basis of the vast diversity of these antibody proteins when somatic recombination of immunoglobulin genes was by Susumu Tonegawa in 1976.[81]
[edit] See also
[edit] References
- ^ Litman GW, Rast JP, Shamblott MJ (1993). "Phylogenetic diversification of immunoglobulin genes and the antibody repertoire". Mol. Biol. Evol. 10 (1): 60'72. PMID 8450761.
- ^ a b c d Eleonora Market, F. Nina Papavasiliou (2003) V(D)J Recombination and the Evolution of the Adaptive Immune System PLoS Biology1(1): e16.
- ^ a b c d e f g h i Janeway CA, Jr et al. (2001). Immunobiology. (5th ed.). Garland Publishing. ISBN 0-8153-3642-X. http://www.ncbi.nlm.nih.gov/books/bv.fcgi?call=bv.View..ShowTOC&rid=imm.TOC&depth=10.
- ^ a b c Rhoades RA, Pflanzer RG (2002). Human Physiology (4th ed.). Thomson Learning. ISBN 0-534-42174-1.
- ^ a b Diaz M, Casali P (2002). "Somatic immunoglobulin hypermutation". Curr Opin Immunol 14 (2): 235'40. doi:10.1016/S0952-7915(02)00327-8. PMID 11869898.
- ^ a b c d e f Pier GB, Lyczak JB, Wetzler LM (2004). Immunology, Infection, and Immunity. ASM Press. ISBN 1-55581-246-5.
- ^ Parker D (1993). "T cell-dependent B cell activation". Annu. Rev. Immunol. 11: 331'60. doi:10.1146/annurev.iy.11.040193.001555. PMID 8476565.
- ^ a b c d Wintrobe, Maxwell Myer (2004). Wintrobe's clinical hematology. John G. Greer, John Foerster, John N Lukens, George M Rodgers, Frixos Paraskevas (11 ed.). Hagerstown, MD: Lippincott Williams & Wilkins. pp. 453'456. ISBN 0-7817-3650-1.
- ^ Tolar P, Sohn HW, Pierce SK (February 2008). "Viewing the antigen-induced initiation of B-cell activation in living cells". Immunol. Rev. 221: 64'76. doi:10.1111/j.1600-065X.2008.00583.x. PMID 18275475. http://www.blackwell-synergy.com/openurl?genre=article&sid=nlm:pubmed&issn=0105-2896&date=2008&volume=221&spage=64.
- ^ Underdown B, Schiff J (1986). "Immunoglobulin A: strategic defense initiative at the mucosal surface". Annu Rev Immunol 4: 389'417. doi:10.1146/annurev.iy.04.040186.002133. PMID 3518747.
- ^ a b Geisberger R, Lamers M, Achatz G (2006). "The riddle of the dual expression of IgM and IgD". Immunology 118 (4): 429'37. doi:10.1111/j.1365-2567.2006.02386.x. PMID 16895553.
- ^ Chen K, Xu W, Wilson M, He B, Miller NW, Bengtén E, Edholm ES, Santini PA, Rath P, Chiu A, Cattalini M, Litzman J, B Bussel J, Huang B, Meini A, Riesbeck K, Cunningham-Rundles C, Plebani A, Cerutti A (2009). "Immunoglobulin D enhances immune surveillance by activating antimicrobial, proinflammatory and B cell-stimulating programs in basophils". Nature Immunology 10 (8): 889'98. doi:10.1038/ni.1748. PMID 19561614.
- ^ a b c d Woof J, Burton D (2004). "Human antibody-Fc receptor interactions illuminated by crystal structures". Nat Rev Immunol 4 (2): 89'99. doi:10.1038/nri1266. PMID 15040582.
- ^ Goding J (1978). "Allotypes of IgM and IgD receptors in the mouse: a probe for lymphocyte differentiation". Contemp Top Immunobiol 8: 203'43. PMID 357078.
- ^ Mattu T, Pleass R, Willis A, Kilian M, Wormald M, Lellouch A, Rudd P, Woof J, Dwek R (1998). "The glycosylation and structure of human serum IgA1, Fab, and Fc regions and the role of N-glycosylation on Fc alpha receptor interactions". J Biol Chem 273 (4): 2260'72. doi:10.1074/jbc.273.4.2260. PMID 9442070.
- ^ Roux K (1999). "Immunoglobulin structure and function as revealed by electron microscopy". Int Arch Allergy Immunol 120 (2): 85'99. doi:10.1159/000024226. PMID 10545762.
- ^ Barclay A (2003). "Membrane proteins with immunoglobulin-like domains--a master superfamily of interaction molecules". Semin Immunol 15 (4): 215'23. doi:10.1016/S1044-5323(03)00047-2. PMID 14690046.
- ^ Putnam FW, Liu YS, Low TL (1979). "Primary structure of a human IgA1 immunoglobulin. IV. Streptococcal IgA1 protease, digestion, Fab and Fc fragments, and the complete amino acid sequence of the alpha 1 heavy chain". J Biol Chem 254 (8): 2865'74. PMID 107164.
- ^ Huber R (1980). "Spatial structure of immunoglobulin molecules". Klin Wochenschr 58 (22): 1217'31. doi:10.1007/BF01478928. PMID 6780722.
- ^ Heyman B (1996). "Complement and Fc-receptors in regulation of the antibody response". Immunol Lett 54 (2-3): 195'9. doi:10.1016/S0165-2478(96)02672-7. PMID 9052877.
- ^ Borghesi L, Milcarek C (2006). "From B cell to plasma cell: regulation of V(D)J recombination and antibody secretion". Immunol Res 36 (1-3): 27'32. doi:10.1385/IR:36:1:27. PMID 17337763.
- ^ a b Ravetch J, Bolland S (2001). "IgG Fc receptors". Annu Rev Immunol 19: 275'90. doi:10.1146/annurev.immunol.19.1.275. PMID 11244038.
- ^ Rus H, Cudrici C, Niculescu F (2005). "The role of the complement system in innate immunity". Immunol Res 33 (2): 103'12. doi:10.1385/IR:33:2:103. PMID 16234578.
- ^ Racaniello, Vincent (2009-10-06). "Natural antibody protects against viral infection". Virology Blog. http://www.virology.ws/2009/10/06/natural-antibody-protects-against-viral-infection/. Retrieved 2010-01-22.
- ^ Milland J, Sandrin MS (December 2006). "ABO blood group and related antigens, natural antibodies and transplantation". Tissue Antigens 68 (6): 459'66. doi:10.1111/j.1399-0039.2006.00721.x. PMID 17176435.
- ^ Mian I, Bradwell A, Olson A (1991). "Structure, function and properties of antibody binding sites". J Mol Biol 217 (1): 133'51. doi:10.1016/0022-2836(91)90617-F. PMID 1988675.
- ^ Fanning LJ, Connor AM, Wu GE (1996). "Development of the immunoglobulin repertoire". Clin. Immunol. Immunopathol. 79 (1): 1'14. doi:10.1006/clin.1996.0044. PMID 8612345.
- ^ a b Nemazee D (2006). "Receptor editing in lymphocyte development and central tolerance". Nat Rev Immunol 6 (10): 728'40. doi:10.1038/nri1939. PMID 16998507.
- ^ Peter Parham. "The Immune System. 2nd ed. Garland Science: New York, 2005. pg.47-62
- ^ Bergman Y, Cedar H (2004). "A stepwise epigenetic process controls immunoglobulin allelic exclusion". Nat Rev Immunol 4 (10): 753'61. doi:10.1038/nri1458. PMID 15459667.
- ^ Honjo T, Habu S (1985). "Origin of immune diversity: genetic variation and selection". Annu Rev Biochem 54: 803'30. doi:10.1146/annurev.bi.54.070185.004103. PMID 3927822.
- ^ a b Or-Guil M, Wittenbrink N, Weiser AA, Schuchhardt J (2007). "Recirculation of germinal center B cells: a multilevel selection strategy for antibody maturation". Immunol. Rev. 216: 130'41. doi:10.1111/j.1600-065X.2007.00507.x. PMID 17367339.
- ^ Neuberger M, Ehrenstein M, Rada C, Sale J, Batista F, Williams G, Milstein C (March 2000). "Memory in the B-cell compartment: antibody affinity maturation". Philos Trans R Soc Lond B Biol Sci 355 (1395): 357'60. doi:10.1098/rstb.2000.0573. PMID 10794054.
- ^ Stavnezer J, Amemiya CT (2004). "Evolution of isotype switching". Semin. Immunol. 16 (4): 257'75. doi:10.1016/j.smim.2004.08.005. PMID 15522624.
- ^ Durandy A (2003). "Activation-induced cytidine deaminase: a dual role in class-switch recombination and somatic hypermutation". Eur. J. Immunol. 33 (8): 2069'73. doi:10.1002/eji.200324133. PMID 12884279.
- ^ Casali P, Zan H (2004). "Class switching and Myc translocation: how does DNA break?". Nat. Immunol. 5 (11): 1101'3. doi:10.1038/ni1104-1101. PMID 15496946.
- ^ Lieber MR, Yu K, Raghavan SC (2006). "Roles of nonhomologous DNA end joining, V(D)J recombination, and class switch recombination in chromosomal translocations". DNA Repair (Amst.) 5 (9-10): 1234'45. doi:10.1016/j.dnarep.2006.05.013. PMID 16793349.
- ^ "Animated depictions of how antibodies are used in ELISA assays". Cellular Technology Ltd.'Europe. http://www.elispot-analyzers.de/english/elisa-animation.html. Retrieved 2007-05-08.
- ^ "Animated depictions of how antibodies are used in ELISPOT assays". Cellular Technology Ltd.'Europe. http://www.elispot-analyzers.de/english/elispot-animation.html. Retrieved 2007-05-08.
- ^ Stern P (2006). "Current possibilities of turbidimetry and nephelometry". Klin Biochem Metab 14 (3): 146'151. http://www.clsjep.cz/odkazy/kbm0603-146.pdf.
- ^ a b Dean, Laura (2005). "Chapter 4: Hemolytic disease of the newborn". Blood Groups and Red Cell Antigens. NCBI Bethesda (MD): National Library of Medicine (US),. http://www.ncbi.nlm.nih.gov/books/bv.fcgi?rid=rbcantigen.chapter.ch4.
- ^ Feldmann M, Maini R (2001). "Anti-TNF alpha therapy of rheumatoid arthritis: what have we learned?". Annu Rev Immunol 19: 163'96. doi:10.1146/annurev.immunol.19.1.163. PMID 11244034.
- ^ Doggrell S (2003). "Is natalizumab a breakthrough in the treatment of multiple sclerosis?". Expert Opin Pharmacother 4 (6): 999'1001. doi:10.1517/14656566.4.6.999. PMID 12783595.
- ^ Krueger G, Langley R, Leonardi C, Yeilding N, Guzzo C, Wang Y, Dooley L, Lebwohl M (2007). "A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis". N Engl J Med 356 (6): 580'92. doi:10.1056/NEJMoa062382. PMID 17287478.
- ^ Plosker G, Figgitt D (2003). "Rituximab: a review of its use in non-Hodgkin's lymphoma and chronic lymphocytic leukaemia". Drugs 63 (8): 803'43. doi:10.2165/00003495-200363080-00005. PMID 12662126.
- ^ Vogel C, Cobleigh M, Tripathy D, Gutheil J, Harris L, Fehrenbacher L, Slamon D, Murphy M, Novotny W, Burchmore M, Shak S, Stewart S (2001). "First-line Herceptin monotherapy in metastatic breast cancer". Oncology 61 Suppl 2: 37'42. doi:10.1159/000055400. PMID 11694786.
- ^ LeBien TW (1 July 2000). "Fates of human B-cell precursors". Blood 96 (1): 9'23. PMID 10891425. http://bloodjournal.hematologylibrary.org/cgi/content/full/96/1/9.
- ^ Ghaffer A (2006-03-26). "Immunization". Immunology - Chapter 14. University of South Carolina School of Medicine. http://pathmicro.med.sc.edu/ghaffar/immunization.htm. Retrieved 2007-06-06.
- ^ Urbaniak S, Greiss M (2000). "RhD haemolytic disease of the fetus and the newborn". Blood Rev 14 (1): 44'61. doi:10.1054/blre.1999.0123. PMID 10805260.
- ^ a b Fung Kee Fung K, Eason E, Crane J, Armson A, De La Ronde S, Farine D, Keenan-Lindsay L, Leduc L, Reid G, Aerde J, Wilson R, Davies G, Désilets V, Summers A, Wyatt P, Young D (2003). "Prevention of Rh alloimmunization". J Obstet Gynaecol Can 25 (9): 765'73. PMID 12970812.
- ^ Tini M, Jewell UR, Camenisch G, Chilov D, Gassmann M (2002). "Generation and application of chicken egg-yolk antibodies". Comp. Biochem. Physiol., Part a Mol. Integr. Physiol. 131 (3): 569'74. doi:10.1016/S1095-6433(01)00508-6. PMID 11867282.
- ^ Cole SP, Campling BG, Atlaw T, Kozbor D, Roder JC (1984). "Human monoclonal antibodies". Mol. Cell. Biochem. 62 (2): 109'20. doi:10.1007/BF00223301. PMID 6087121.
- ^ Kabir S (2002). "Immunoglobulin purification by affinity chromatography using protein A mimetic ligands prepared by combinatorial chemical synthesis". Immunol Invest 31 (3-4): 263'78. doi:10.1081/IMM-120016245. PMID 12472184.
- ^ a b Brehm-Stecher B, Johnson E (2004). "Single-cell microbiology: tools, technologies, and applications". Microbiol Mol Biol Rev 68 (3): 538'59. doi:10.1128/MMBR.68.3.538-559.2004. PMID 15353569. PMC 515252. http://mmbr.asm.org/cgi/content/full/68/3/538?view=long&pmid=15353569.
- ^ Williams N (2000). "Immunoprecipitation procedures". Methods Cell Biol 62: 449'53. doi:10.1016/S0091-679X(08)61549-6. PMID 10503210.
- ^ Kurien B, Scofield R (2006). "Western blotting". Methods 38 (4): 283'93. doi:10.1016/j.ymeth.2005.11.007. PMID 16483794.
- ^ Scanziani E (1998). "Immunohistochemical staining of fixed tissues". Methods Mol Biol 104: 133'40. doi:10.1385/0-89603-525-5:133. PMID 9711649.
- ^ Reen DJ. (1994). "Enzyme-linked immunosorbent assay (ELISA)". Methods Mol Biol. 32: 461'6. doi:10.1385/0-89603-268-X:461. PMID 7951745.
- ^ Kalyuzhny AE (2005). "Chemistry and biology of the ELISPOT assay". Methods Mol Biol. 302: 15'31. doi:10.1385/1-59259-903-6:015. PMID 15937343.
- ^ Whitelegg N.R.J., Rees A.R. (2000). "WAM: an improved algorithm for modeling antibodies on the WEB". Protein Engineering 13 (12): 819'824. doi:10.1093/protein/13.12.819. PMID 11239080. http://peds.oxfordjournals.org/cgi/content/abstract/13/12/819.
WAM - ^ Marcatili P, Rosi A,Tramontano A (2008). "PIGS: automatic prediction of antibody structures". Bioinformatics 24 (17): 1953'1954. doi:10.1093/bioinformatics/btn341. PMID 18641403.
Prediction of Immunoglobulin Structure (PIGS) - ^ Sivasubramanian A, Sircar A, Chaudhury S, Gray J J (2009). "Toward high-resolution homology modeling of antibody Fv regions and application to antibody'antigen docking". Proteins 74 (2): 497'514. doi:10.1002/prot.22309. PMID 19062174.
RosettaAntibody - ^ a b c Lindenmann, Jean (1984). "Origin of the Terms 'Antibody' and 'Antigen'". Scand. J. Immunol. 19 (4): 281'5. PMID 6374880. http://www3.interscience.wiley.com/cgi-bin/fulltext/119531625/PDFSTART. Retrieved 2008-11-01.
- ^ Padlan, Eduardo (February 1994). "Anatomy of the antibody molecule". Mol. Immunol. 31(3): 169'217. doi:10.1016/0161-5890(94)90001-9.
- ^ "New Sculpture Portraying Human Antibody as Protective Angel Installed on Scripps Florida Campus". http://www.scripps.edu/newsandviews/e_20081110/sculpture.html. Retrieved 2008-12-12.
- ^ "Protein sculpture inspired by Vitruvian Man". http://www.boingboing.net/2008/10/22/protein-sculpture-in.html. Retrieved 2008-12-12.
- ^ "Emil von Behring - Biography". http://nobelprize.org/nobel_prizes/medicine/laureates/1901/behring-bio.html. Retrieved 2007-06-05.
- ^ AGN (1931). "The Late Baron Shibasaburo Kitasato" ([dead link]). Canadian Medical Association Journal: 206. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=382621_prizes/medicine/laureates/1901/behring-bio.html.
- ^ Winau F, Westphal O, Winau R (2004). "Paul Ehrlich--in search of the magic bullet". Microbes Infect. 6 (8): 786'9. doi:10.1016/j.micinf.2004.04.003. PMID 15207826.
- ^ Silverstein AM (2003). "Cellular versus humoral immunology: a century-long dispute". Nat. Immunol. 4 (5): 425'8. doi:10.1038/ni0503-425. PMID 12719732.
- ^ Van Epps HL (2006). "Michael Heidelberger and the demystification of antibodies". J. Exp. Med. 203 (1): 5. doi:10.1084/jem.2031fta. PMID 16523537. PMC 2118068. http://www.jem.org/cgi/reprint/203/1/5.pdf.
- ^ Marrack, JR (1938). Chemistry of antigens and antibodies (2nd ed.). London: His Majesty's Stationery Office. OCLC 3220539.
- ^ "The Linus Pauling Papers: How Antibodies and Enzymes Work". http://profiles.nlm.nih.gov/MM/Views/Exhibit/narrative/specificity.html. Retrieved 2007-06-05.
- ^ Silverstein AM (2004). "Labeled antigens and antibodies: the evolution of magic markers and magic bullets". Nat. Immunol. 5 (12): 1211'7. doi:10.1038/ni1140. PMID 15549122. http://users.path.ox.ac.uk/~seminars/halelibrary/Paper%2018.pdf.
- ^ Edelman GM, Gally JA (1962). "The nature of Bence-Jones proteins. Chemical similarities to polypetide chains of myeloma globulins and normal gamma-globulins". J. Exp. Med. 116: 207'27. doi:10.1084/jem.116.2.207. PMID 13889153.
- ^ Stevens FJ, Solomon A, Schiffer M (1991). "Bence Jones proteins: a powerful tool for the fundamental study of protein chemistry and pathophysiology". Biochemistry 30 (28): 6803'5. doi:10.1021/bi00242a001. PMID 2069946.
- ^ a b Raju TN (1999). "The Nobel chronicles. 1972: Gerald M Edelman (b 1929) and Rodney R Porter (1917-85)". Lancet 354 (9183): 1040. PMID 10501404.
- ^ Tomasi TB (1992). "The discovery of secretory IgA and the mucosal immune system". Immunol. Today 13 (10): 416'8. doi:10.1016/0167-5699(92)90093-M. PMID 1343085.
- ^ Preud'homme JL, Petit I, Barra A, Morel F, Lecron JC, Lelièvre E (2000). "Structural and functional properties of membrane and secreted IgD". Mol. Immunol. 37 (15): 871'87. doi:10.1016/S0161-5890(01)00006-2. PMID 11282392.
- ^ Johansson SG (2006). "The discovery of immunoglobulin E". Allergy and asthma proceedings : the official journal of regional and state allergy societies 27 (2 Suppl 1): S3'6. PMID 16722325.
- ^ Hozumi N, Tonegawa S (1976). "Evidence for somatic rearrangement of immunoglobulin genes coding for variable and constant regions". Proc. Natl. Acad. Sci. U.S.A. 73 (10): 3628'32. doi:10.1073/pnas.73.10.3628. PMID 824647.
[edit] External links
| Wikimedia Commons has media related to: Antibodies |
- Mike's Immunoglobulin Structure/Function Page at University of Cambridge
- Antibodies as the PDB molecule of the month Discussion of the structure of antibodies at Protein Data Bank
- Microbiology and Immunology On-line Textbook at University of South Carolina
- A hundred years of antibody therapy History and applications of antibodies in the treatment of disease at University of Oxford
- How Lymphocytes Produce Antibody from Cells Alive!
- Antibody applications Fluorescent antibody image library, University of Birmingham
|
||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||
|
|||||||||||||||||||||||||
|
SOURCES.COM is an online portal and directory for journalists, news media, researchers and anyone seeking experts, spokespersons, and reliable information resources. Use SOURCES.COM to find experts, media contacts, news releases, background information, scientists, officials, speakers, newsmakers, spokespeople, talk show guests, story ideas, research studies, databases, universities, associations and NGOs, businesses, government spokespeople. Indexing and search applications by Ulli Diemer and Chris DeFreitas.
For information about being included in SOURCES as a expert or spokesperson see the FAQ . For partnerships, content and applications, and domain name opportunities contact us.